Opinion Article
Volume 3 Issue 2 - 2020
ATPCI: Mission Impossible
1St. John of God Hospital, Department of Cardiology, Budapest, Hungary
2György Gottsegen National Institute of Cardiology, Budapest, Hungary
2György Gottsegen National Institute of Cardiology, Budapest, Hungary
*Corresponding Author: János Tomcsányi, MD, PhD, Department of Cardiology, St John of God Hospital, Budapest, Hungary.
We read with great interest the results of ATPCI, a randomized, double-blind placebo-controlled clinical trial about the efficacy and safety of trimetazidine after coronary intervention. [1]
The trial published by Ferrari et al. indicated that trimetazidine had a neutral effect compared to placebo. This result is somewhat contradictory to what was observed in a prior study of Xu et al.. The latter was a prospective study with two years of follow up, which found that trimetazidine significantly reduces recurring angina after DES implantation in elderly diabetic patients. [2]
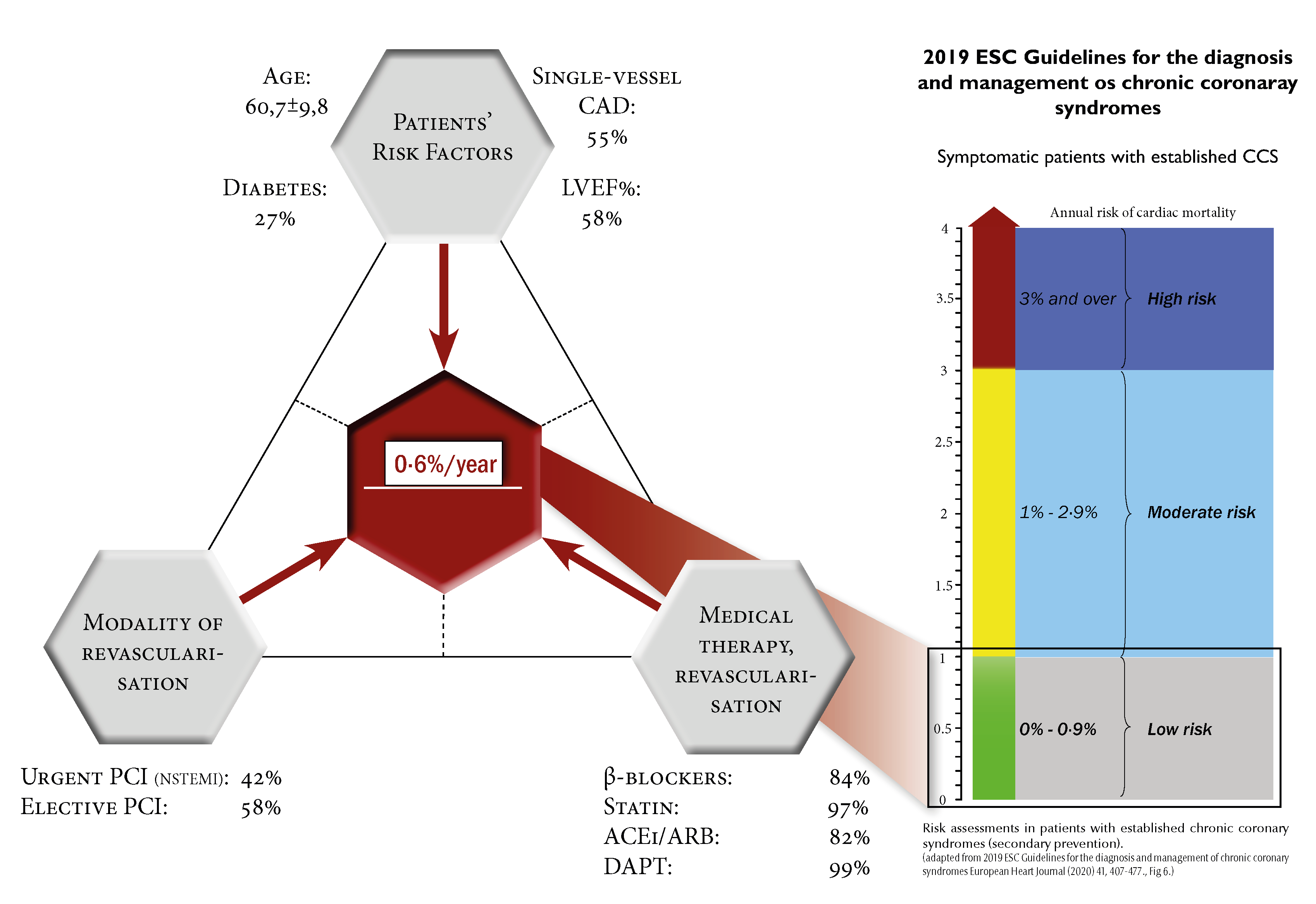
The differing characteristics of the patient populations enrolled in the two studies may explain this apparent contradiction. ATPCI was a study of young patients with good left ventricular function who were on optimal medical therapy and in whom successful revascularization was achieved. Of note, more than half of the patients enrolled in this study had single vessel disease and the enrolment criteria did not include diabetes.
Given the patient characteristics, the low average annual cardiac mortality of 0.6% was not surprising (Figure 1). However, an added benefit cannot be expected of any medication in a low-risk population with such a low rate of cardiac death.
A similar problem was highlighted regarding the Reminder study, in which the efficacy of eplerenone was investigated after revascularisation in STEMI in patients with preserved left ventricular function and a very low CV mortality of 0.4% in the placebo arm. [3]
ATPCI simply confirms the previous observation that it is not just the presence of coronary artery disease and its type but also the patients’ risk status that determines the outcome.
Risk status is determined by age, revascularization status, left ventricular systolic function and optimized medical therapy as indicated by the results of the TIGRIS registry [4] and the COURAGE risk prediction follow-up study.[5]
Competing Interests:
We declare no competing interests.
References
- Roberto Ferrari., et al. “Efficacy and safety of trimetazidine after percutaneous coronary intervention (ATPCI): a randomised, double-blind, placebo-controlled trial”. Lancet 396.10254 (2020): 830-838.
- Xiaohan Xu., et al. “Effect of Trimetazidine on Recurrent Angina Pectoris and Left Ventricular Structure in Elderly Multivessel Coronary Heart Disease Patients with Diabetes Mellitus After Drug-Eluting Stent Implantation: A Single-Centre, Prospective, Randomized, Double-Blind Study at 2-Year Follow-Up”. Clinical Drug Investigation 34 (2014): 251-258.
- Gilles Montalescot., et al. “Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: The Randomized Double-Blind Reminder Study”. European Heart Journal 35.34 (2014): 2295-2302.
- David Brieger., et al. “Two-year outcomes among stable high-risk patients following acute MI. Insights from a global registry in 25 countries”. International Journal of Cardiology 311 (2020): 7-14.
- William E.Boden., et al. “Risk Prediction Tool for Assessing the Probability of Death or Myocardial Infarction in Patients With Stable Coronary Artery Disease”. The American Journal of Cardiology 130.1(2020): 1-6.
Citation:
János Tomcsányi., et al. “ATPCI: Mission Impossible”. Therapeutic Advances in Cardiology 3.1 (2020): 16-17.
Copyright: © 2020 János Tomcsányi., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.